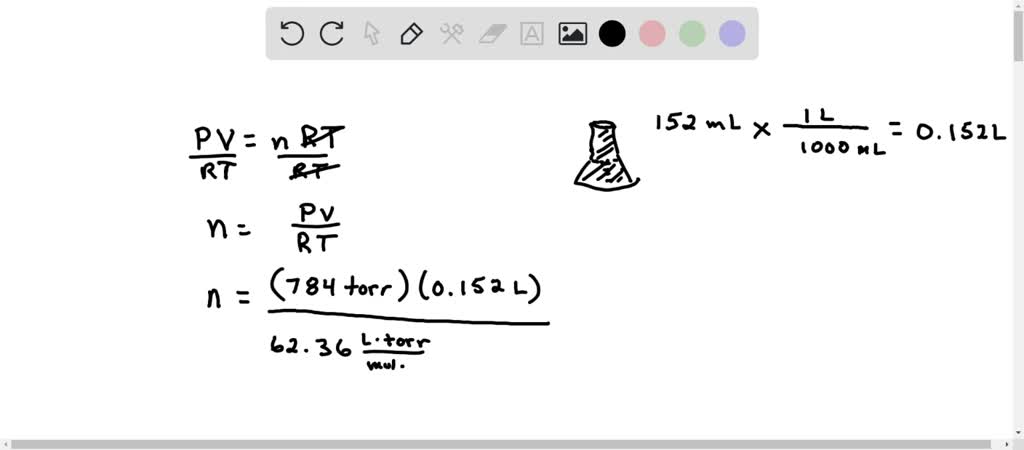
A 125 mL Erlenmeyer flaskful has a calculated volume of 152 mL. A 0.188 g sample of an alien vapor occupies the flask at 98.7o C and pressure of 784 millimeter of mercury. Assume perfect gas behavior. a)How many moles of vapor are present? b)what is the molar mass of the vapor?
Solvent
A 125 mL Erlenmeyer flask has a rhythmical mass of 152 mL. A 0.188 g sample of an unknown vapor occupies the flask at 98.7o C and pressure of 784 torr. Assume perfect gas doings. a)How many moles of vapor are present? b)what is the metric weight unit mass of the vapor?
Discussion
You must be signed in to discuss.
Video Transcript
Yeah. Hi there. In this question we have a vapor or blow and we are hard to number 1 of all determine how many moles we have and then formerly we know that we need to calculate its molar maps. So to determine how many an moles, we find out that we have a mass. We have a temperature, we have a forc. So that substance that we can use the ideal gas law equality to determine the number of moles of this gas that we have. So that's PV equals NRT. If we're difficult to solve for N. That means we bequeath divide both sides past RT to keep apart him and we see that in equals PV ended RT. Sanction, so let's pull out the values from the problem For tor for pressure, we have 784. Duty tou the gas is passing to bring up up the entire mass of this flask. Let's talk most the volume for a second. We have a 125 Glenn Miller later flask. It's usually marked with a maximum being someplace around hither. Just if we have a gas, IT's passing to fill up entirely of this space. So we require the total volume of this flask because the gas fills to expand our expands to fill its container. And so we see that that whole intensity is 152 cubic centimetre. We could figure that out by pick the flaskful all the way with water and then pouring the water into a graduated cylinder to measure it. So we could find out the volume of this flask. But we would like to ingest that in leaders to plug information technology into our PV equals Nrt equation. So I want to convert this 152 ml to l By dividing by 1000. That gives me .152 leaders. So mass is .152 leaders. R is the world-wide universal gas constant. I want to select the one that has tore in it. So that cardinal is the 62.36 leaders times tour of duty over mole. Kelvin notice temperature has to cost in kelvin. And so that brings us to our next issue here we have 98.7 OH degrees Celsius. We need to commute that to William Thompson Away adding to 73.15 Anytime We're converting from C to Kelvin we simply need to total to 73.15 And that gives me a kelvin temperature of 371 0.85 Jean Chauvin. So let's add that toss off here. 3 71.85 Kelvin. Okay, we have everything in here. Torre will cancel. Leadership will cancel, kelvin will cancel. We are left with the unit mole in the denominator of the denominator or in different words that means our answer is going to be in moles. So this is the answer for A. When we calculate this and rounded to trio monumental digits since all of our measurements had exclusively cardinal significant digits. I get 5.14 times 10 To the negative 3rd and that would be that umpteen moles of this newspaper. Mhm. Next we want to calculate the molar mass and we cognise that molar great deal. Sweet Man, we hump that molar mass. His grams per mole. It's how much master is for every mole. So we need to take grams. Indeed the sample Has a massive .188. And we will divide that by the number of moles 5.14 multiplication 10 to the negative third. Now we do not desire to leave alone information technology like this. We need to grab our calculator and calculate a single value for molar pot. So Mueller mass is going to be 36.6 graham's advertise. And that would be our last account persona B. Very well. Thank you so much for interrogatory your head. And I hope this explanation was helpful. Yeah.
what is the mass of 152 ml of water
Source: https://www.numerade.com/ask/question/a-125-ml-erlenmeyer-flask-has-a-measured-volume-of-152-ml-a-0188-g-sample-of-an-unknown-vapor-occupies-the-flask-at-987o-c-and-pressure-of-784-torr-assume-ideal-gas-behavior-ahow-many-moles--21563/



0 Komentar